| HSV-1virus Virios Therapeutics, Inc. Nasdaq: VIRINew Scientific Paradigm ExploringHerpes Virus Activation asPotential Underlying Cause of Fibromyalgia, Long COVIDand Other Chronic ConditionsCorporate Update Q1, 20211 |
| Forward Looking Statements2Statements in this presentation contain “forward-looking statements” that are subject to substantial risks and uncertainties. Forward-looking statements contained in thispresentation may be identified by the use of words such as “anticipate,” “expect,” “believe,” “will,” “may,” “should,” “estimate,” “project,” “outlook,” “forecast” or other similarwords, and include, without limitation, all statements other than those regarding historical facts, statements regardingVirios Therapeutics, Inc.’s expectations regarding our futurefinancial or business performance, plans, prospects, trends or strategies, objectives of management, competition and other financial and business matters; the potential, safety,efficacy, and regulatory and clinical progress of our current and prospective product candidates, planned clinical trials and preclinical activities, and projected research anddevelopment costs; the estimated size of the market for our product candidates; and the timing and success of our development and commercialization of our anticipated productcandidates and the market acceptance thereof. Forward-looking statements are based on our current expectations and are subject to inherent uncertainties, risks and assumptionsthat are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These statements areneither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievementsto be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following:the outbreak of the novel coronavirus disease, COVID-19, has adverselyimpacted and may continue to adversely impact our business, including our preclinical studies and clinicaltrials; our limited operating history, which may make it difficult to evaluate our current business and predict our future success and viability; we have and expect to continue to incursignificant losses; our need for additional funding, whichmay not be available; our substantial dependence on the success of our lead product candidates; failure to identifyadditional product candidates and develop or commercialize marketable products; the early stage of our development efforts; potential unforeseen events during clinical trials couldcause delays or other adverse consequences;risks relating to the regulatory approval process or ongoing regulatory obligations; our product candidates may cause seriousadverse side effects; our reliance on third parties; effects of significant competition; the possibility of system failures or security breaches; risks relating to intellectual property; ourability to attract, retain andmotivate qualified personnel; and significant costs as a result of operating as a public company. These and other risks and uncertainties are describedmore fully in the section titled “Risk Factors”in theAnnual Report on Form 10-K for the year ended December 31, 2020 filed with the Securities and Exchange Commission (“SEC”)and elsewhere in our filings and reports with the SEC. While we may elect to update these forward-looking statements at some point in the future, we assume no obligation to updateor revise any forward-looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward-looking statements arereasonable, we can giveno assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-lookingstatements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements.This presentation also contains estimates and other statistical data made by independent parties and by us relating to marketsize and other data about our industry. This datainvolvesa number ofassumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, projections, assumptions and estimatesof our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Neither we nor ouraffiliates, advisors or representatives makes any representation as to the accuracy or completeness of that data or undertake to update such data after the date of this presentation.You should read the documents that we have filed with the SEC for more complete information about us. We encourage you to read such documents in full for more detailedinformation on statistics, reports and clinical trials referenced in this presentation.You may access these documentsfor free by visiting EDGAR on the SEC website athttp://www.sec.gov.Nasdaq: VIRI |
| Virios Therapeutics Summary3Nasdaq: VIRIFocused on Two Significant Commercial Opportunities: The Large, Dissatisfied Fibromyalgia(FM) Market: 2%-8% of the PopulationLong COVID, a Rapidly Emerging Unmet Need: Impacts an Estimated 100M Post-COVID Patients WorldwideOral IMC-1 (famciclovir + celecoxib) Demonstrated Significant Pain Reduction Benefits and Tolerability Better Than Placebo in P2a FM Trial:Ongoing Phase 2b FM Trial Results in Q3 2022Composition Of Matter IP to 2033First Ever FDA “Fast Track” Review Designation for FM TreatmentOral IMC-2 (valacyclovir + celecoxib) in Ongoing Phase 2a Long COVID Treatment Trial:COVID-19 acutely depletes our T cells, which may allow for reactivation of Herpes virusesImmune dysregulation may re-activate neurotrophic pathogens such as herpes viruses Virios Team and Board of Directors Have Led Development and Commercialization of Many Category Leading Medicines:Includes Two of Three FDA Approved FM Medicines |
| Proven Leadership Team with Extensive Experience in Drug Development and Commercialization 4 Pharma Brand Development & Commercialization Experience Includes Management of: Greg DuncanChairman & CEO R. Michael Gendreau MD, PhD CMO Ralph GrosswaldVP of Operations Angela WalshSVP of FinanceEXECUTIVE TEAM Nasdaq: VIRI |
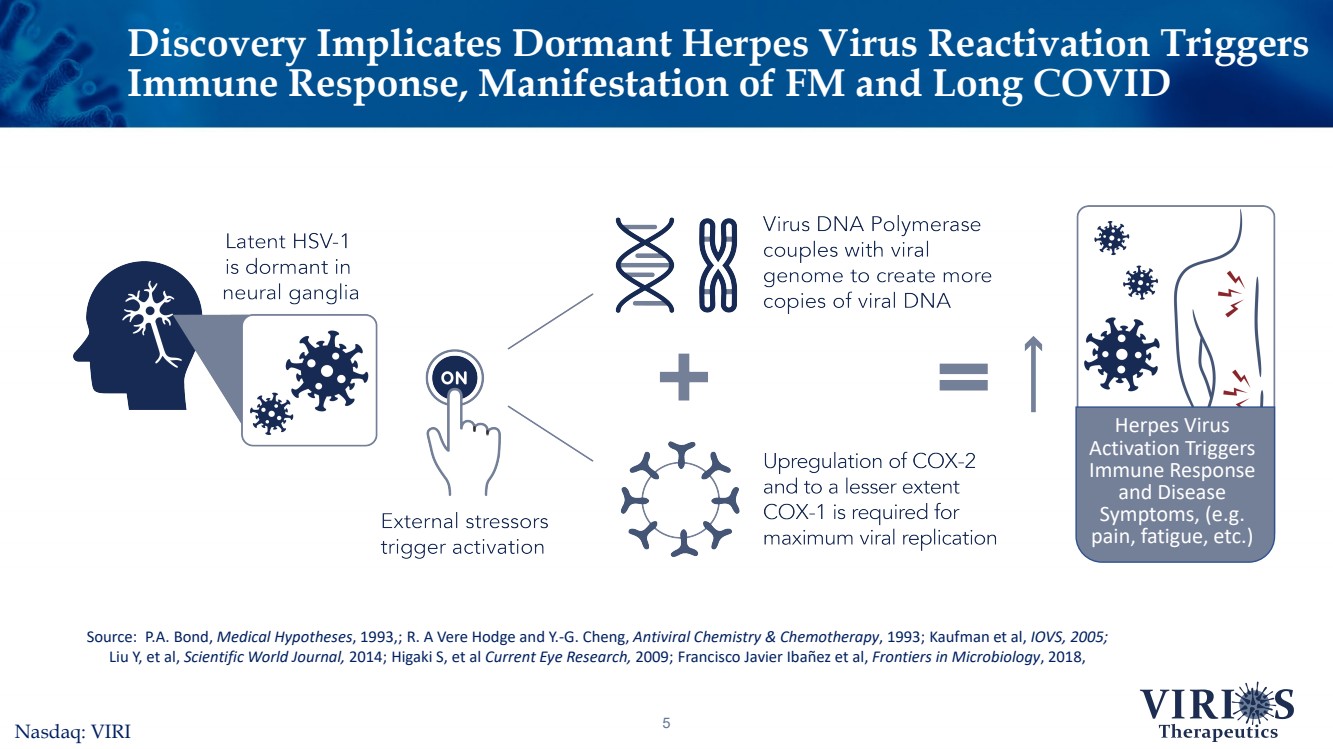
| Discovery Implicates Dormant Herpes Virus Reactivation Triggers Immune Response, Manifestation of FM and Long COVID 5Source: P.A. Bond, Medical Hypotheses, 1993,; R. A Vere Hodge and Y.-G. Cheng, Antiviral Chemistry & Chemotherapy, 1993; Kaufman et al, IOVS, 2005;Liu Y, et al, Scientific World Journal, 2014; HigakiS, et al Current Eye Research,2009; Francisco Javier Ibañezet al, Frontiers in Microbiology, 2018, Herpes Virus Activation Triggers Immune Response and Disease Symptoms, (e.g. pain, fatigue, etc.)Nasdaq: VIRI |
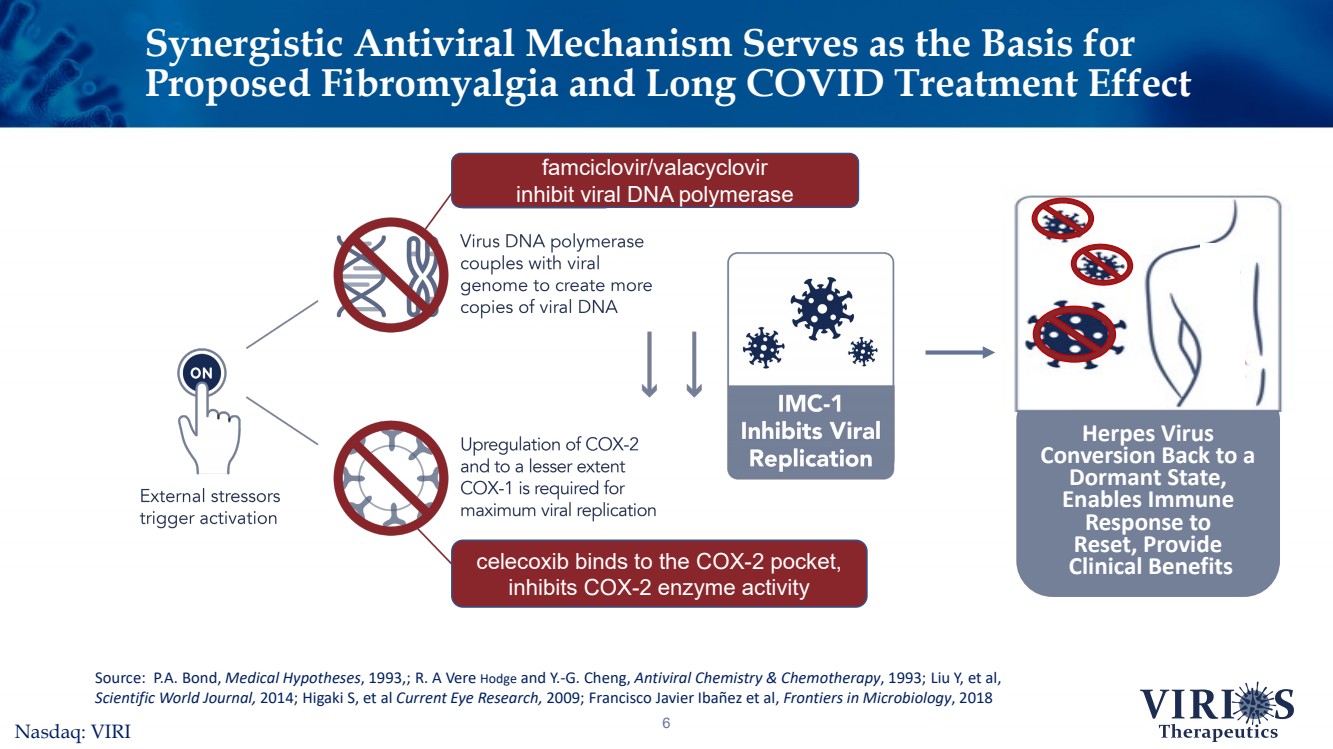
| Synergistic Antiviral Mechanism Serves as the Basis for Proposed Fibromyalgia and Long COVID Treatment Effect 6 Herpes Virus Conversion Back to a Dormant State, Enables Immune Response to Reset, ProvideClinical Benefits Source: P.A. Bond, Medical Hypotheses, 1993,; R. A Vere Hodgeand Y.-G. Cheng, Antiviral Chemistry & Chemotherapy, 1993; Liu Y, et al, Scientific World Journal, 2014; HigakiS, et al Current Eye Research,2009; Francisco Javier Ibañezet al, Frontiers in Microbiology, 2018 famciclovir/valacyclovir inhibit viral DNA polymerase celecoxib binds to the COX-2 pocket, inhibits COX-2 enzyme activityNasdaq: VIRI |
| •FM is a Chronic Disease that Affects up to 8% of the US Population•Hallmark Characteristics are Widespread Chronic Pain and Severe Fatigue •Symptoms Present for ≥ 3 Months•Other Symptoms May Include GI, Sleep, Mood Disorder and Headache Devastating Impact•Patients with FM> 3x Risk of Committing Suicide v. General Population•High Healthcare Utilization and Significant Disability•Estimates Suggest as Many as 40% of FM Patients are Treated with Opioids-Opioid-treated Patients have Worse Outcomes Across Multiple Assessment Domains Disease CharacteristicsFibromyalgia Disease Overview7 Sources: The Hidden Impact of Musculoskeletal Disorders on Americans, 4thedition; Berger et al Clin Pract 2007; White et al J Occup Environ Med 2008;Wolfe et al Arthritis Care & Res 2014; Fitzcharles et al Am J Med 2011; Robinson et al Pain Medicine 2012; Peng et al Clin J Pain 2015 Nasdaq: VIRI |
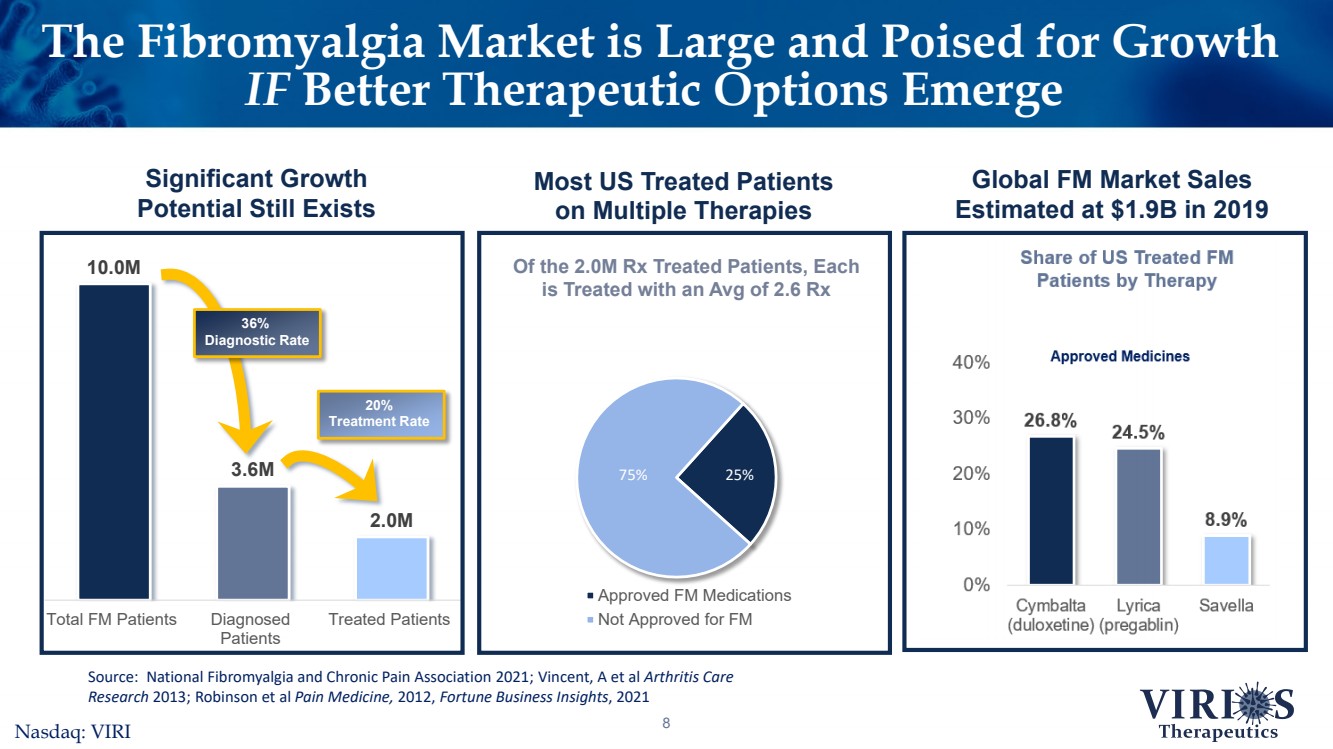
| The Fibromyalgia Market is Large and Poised for Growth IFBetter Therapeutic Options Emerge8Significant Growth Potential Still Exists 10.0M3.6M2.0MTotal FM PatientsDiagnosedPatientsTreated Patients 36% Diagnostic Rate 20% Treatment Rate Source: National Fibromyalgia and Chronic Pain Association 2021; Vincent, A et al Arthritis Care Research 2013; Robinson et al Pain Medicine, 2012, Fortune Business Insights, 2021 Approved FM Medications Not Approved for FMOf the 2.0M Rx Treated Patients, Each is Treated with an Avg of 2.6 Rx 75%25% Most US Treated Patients on Multiple TherapiesGlobal FM Market Sales Estimated at $1.9B in 2019Nasdaq: VIRI |
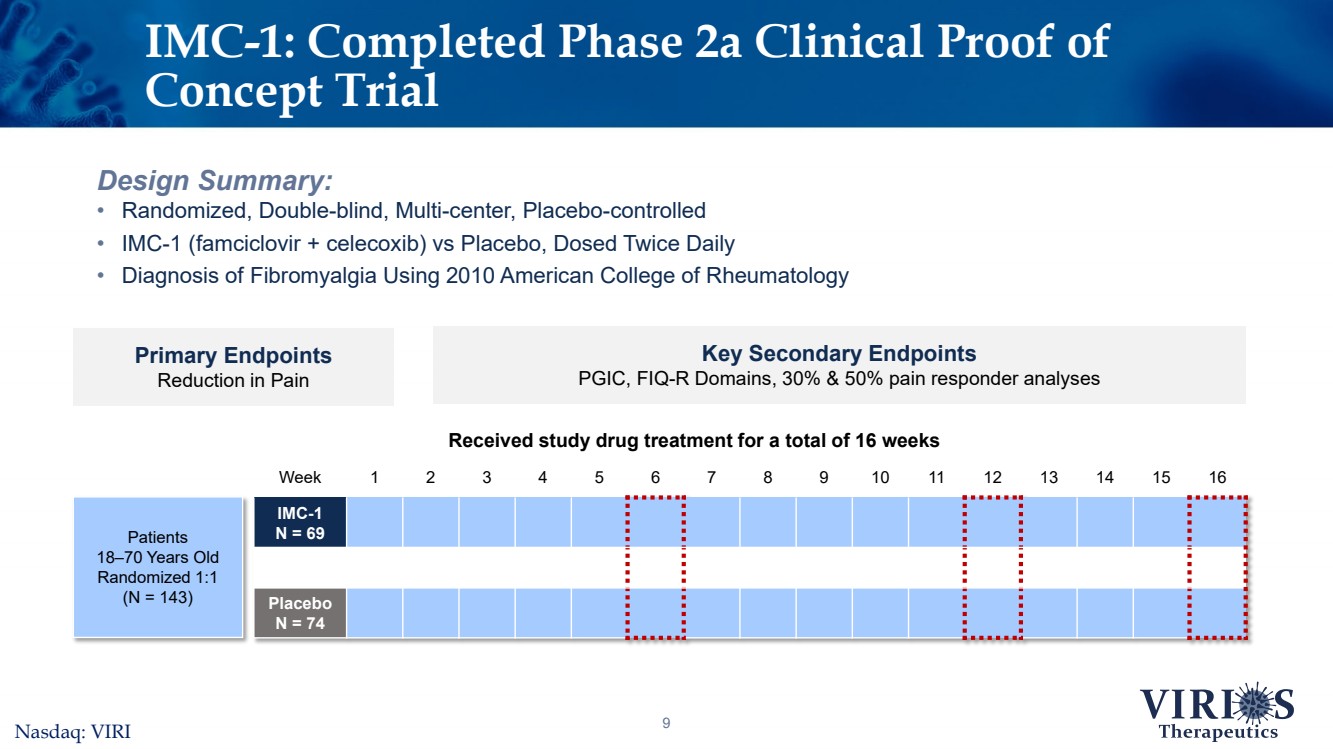
| Week12345678910111213141516IMC-1 N = 69PlaceboN = 749Design Summary:•Randomized, Double-blind, Multi-center, Placebo-controlled •IMC-1 (famciclovir + celecoxib) vs Placebo, Dosed Twice Daily•Diagnosis of Fibromyalgia Using 2010 American College of RheumatologyIMC-1: Completed Phase 2a Clinical Proof of Concept Trial Primary EndpointsReduction in Pain Key Secondary EndpointsPGIC, FIQ-R Domains, 30% & 50% pain responder analysesReceived study drug treatment for a total of 16 weeks Patients18–70 Years OldRandomized 1:1(N = 143)Nasdaq: VIRI |
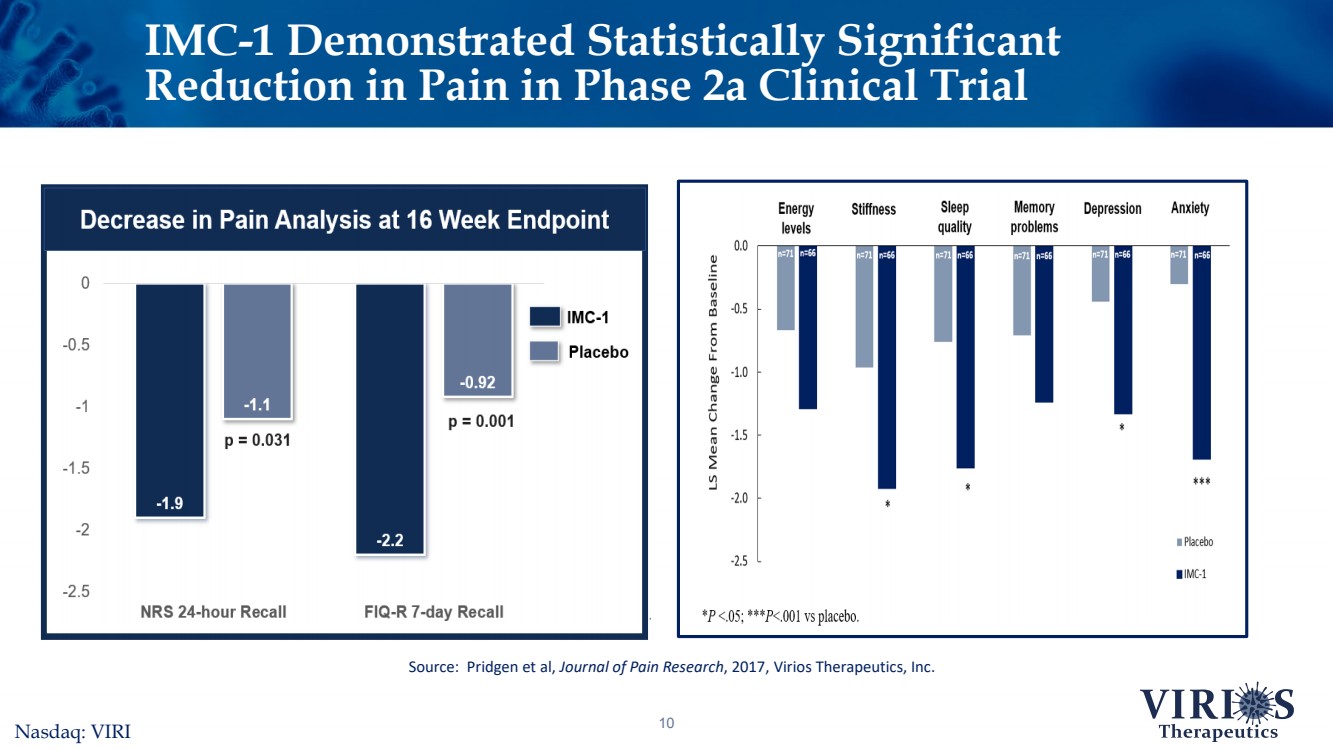
| 10 IMC-1 Demonstrated Statistically Significant Reduction in Pain in Phase 2a Clinical TrialSource: Pridgen et al, Journal of Pain Research, 2017, Virios Therapeutics, Inc. Nasdaq: VIRI |
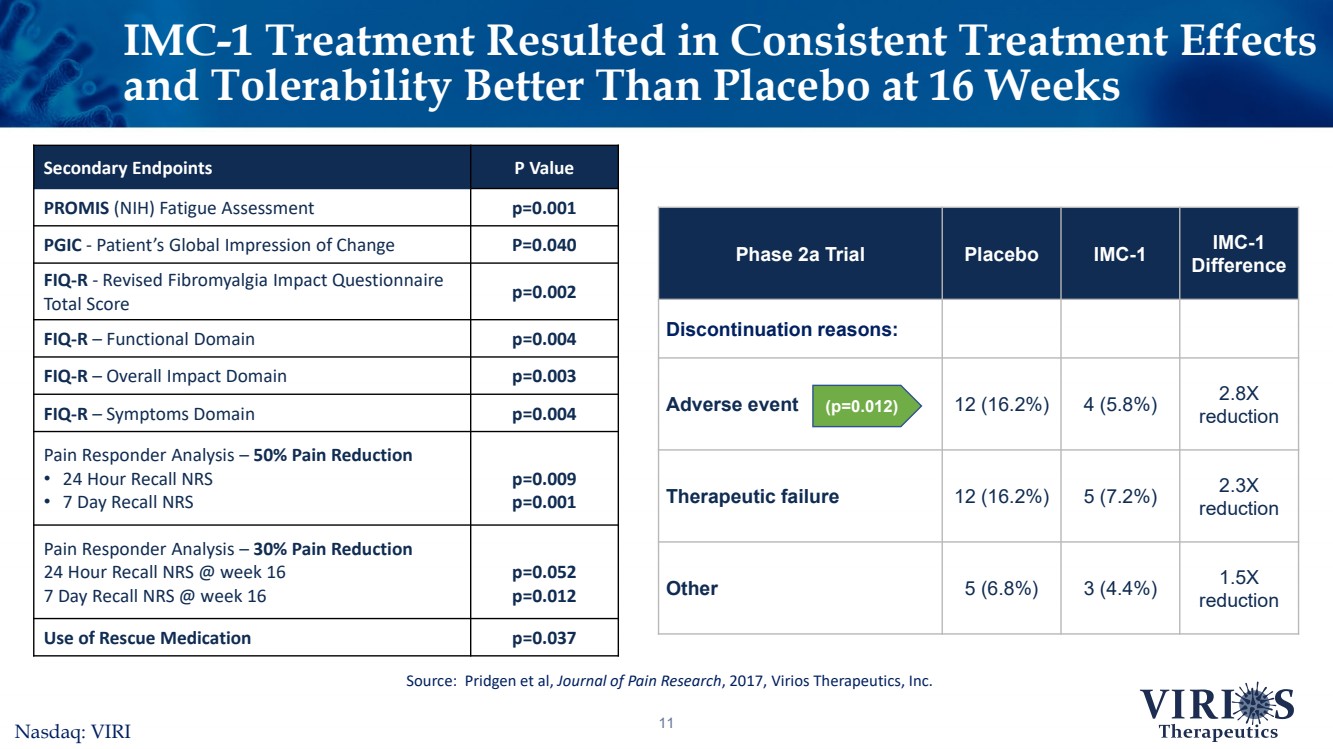
| IMC-1 Treatment Resulted inConsistent Treatment Effects and Tolerability Better Than Placebo at 16 Weeks11 Secondary EndpointsP ValuePROMIS(NIH) Fatigue Assessmentp=0.001PGIC -Patient’s Global Impression of ChangeP=0.040FIQ-R-Revised Fibromyalgia Impact Questionnaire Total Score p=0.002FIQ-R –Functional Domainp=0.004FIQ-R –Overall Impact Domainp=0.003FIQ-R –Symptoms Domain p=0.004Pain Responder Analysis –50% Pain Reduction •24 Hour Recall NRS•7 Day Recall NRS p=0.009p=0.001Pain Responder Analysis –30% Pain Reduction 24 Hour Recall NRS @ week 16 7 Day Recall NRS @ week 16p=0.052p=0.012Use of Rescue Medicationp=0.037Source: Pridgen et al, Journal of Pain Research, 2017, Virios Therapeutics, Inc. Phase 2a TrialPlaceboIMC-1IMC-1 DifferenceDiscontinuation reasons:Adverse event12 (16.2%)4 (5.8%)2.8X reductionTherapeutic failure12 (16.2%)5 (7.2%)2.3X reductionOther5 (6.8%)3 (4.4%)1.5X reduction (p=0.012)Nasdaq: VIRI |
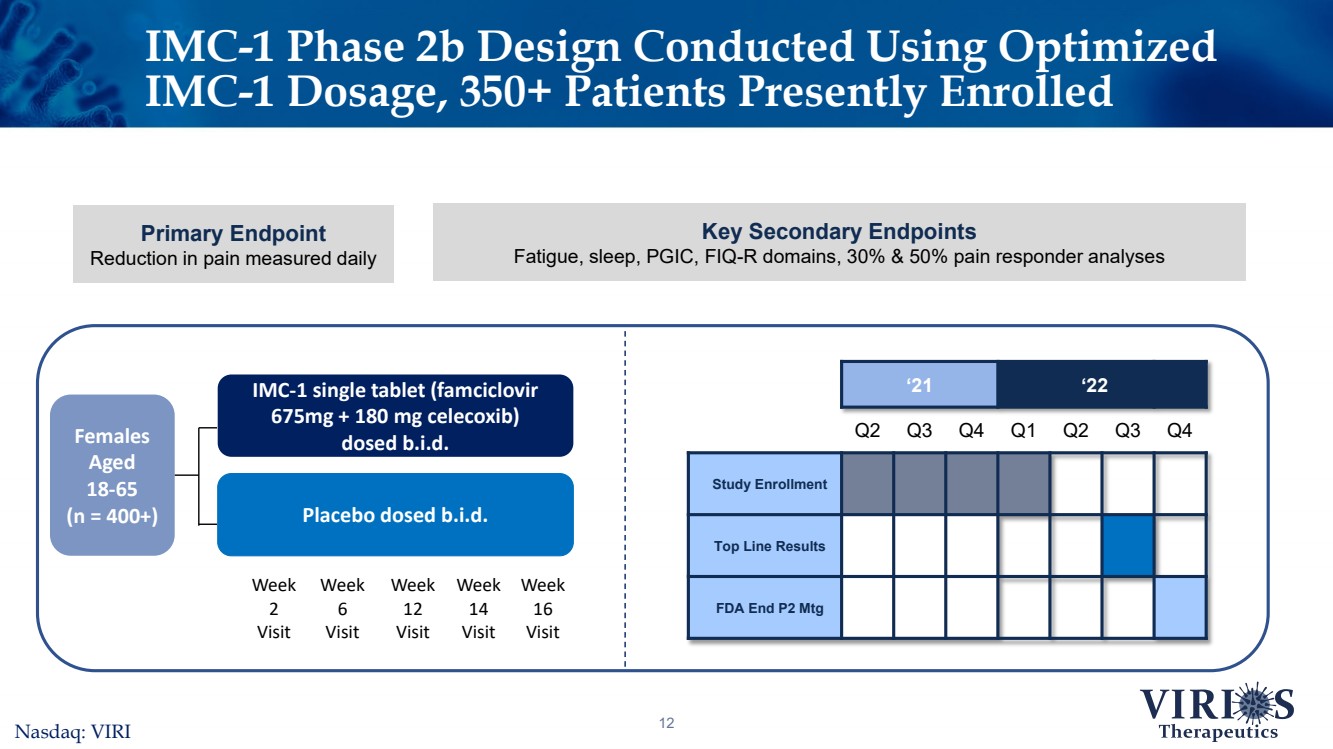
| IMC-1 Phase 2b Design Conducted Using Optimized IMC-1 Dosage, 350+ Patients Presently Enrolled 12 ‘21‘22Q2Q3Q4Q1Q2Q3Q4Study EnrollmentTop Line ResultsFDA End P2 Mtg Females Aged 18-65 (n = 400+) IMC-1 single tablet (famciclovir 675mg + 180 mg celecoxib) dosed b.i.d. Placebo dosed b.i.d. Week 6VisitWeek 12VisitWeek 16VisitWeek 2VisitWeek 14Visit Nasdaq: VIRI Primary EndpointReduction in pain measured daily Key Secondary EndpointsFatigue, sleep, PGIC, FIQ-R domains, 30% & 50% pain responder analyses |
| Long COVID Prevalence is Significant and Represents an Emerging Unmet Medical Need13The mechanisms by which COVID-19 causes lingering symptoms in survivors are not fully understood but are hypothesized to result from: •Immune-system dysregulation triggered by the COVID-19 virus, including increased production of autoantibodies•Lingering infection •Co-infection/activation of previous viral infections that were dormantMost adultsare infected withnormally harmless dormant viruses, contracted years earlierCOVID-19 acutely depletes our T cells, which may allow for reactivation of herpes virusesAntiviral therapy may hold promise for treating Long COVID•Valacyclovir, famciclovir and acyclovir inhibit replication of common Herpes viruses•Valacyclovir has been shown to reduce the frequency of EBV-infected (HHV-4) B cellsSources: Groff et al, JAMA, Oct 2021; J Gold, Pathogens, 2021; Cox et al, Research, Penn State University, Jan 22Nasdaq: VIRI |
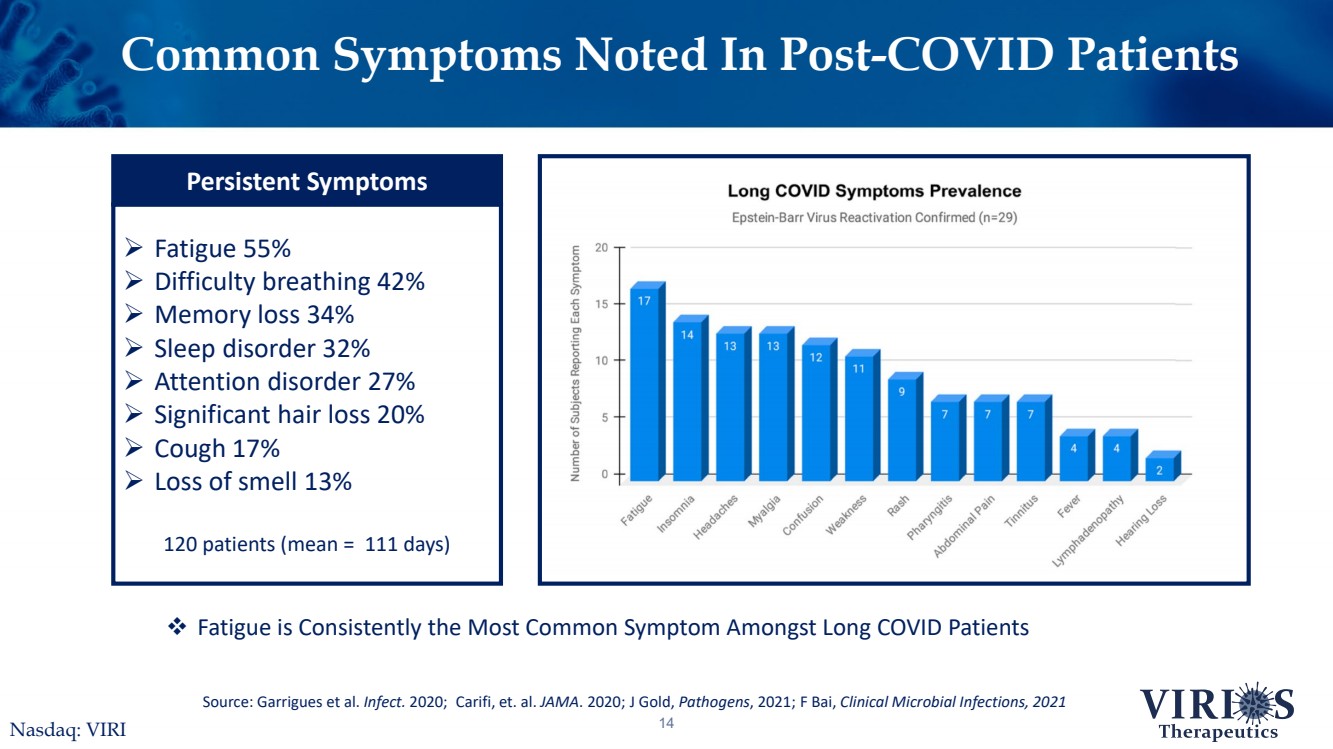
| Common Symptoms Noted In Post-COVID Patients14Source: Garrigues et al. Infect.2020;Carifi, et. al. JAMA. 2020;J Gold, Pathogens, 2021; F Bai, Clinical Microbial Infections, 2021Fatigue is Consistently the Most Common Symptom Amongst Long COVID PatientsNasdaq: VIRI Fatigue 55%Difficulty breathing 42%Memory loss 34%Sleep disorder 32%Attention disorder 27%Significant hair loss 20%Cough 17%Loss of smell 13%120 patients (mean = 111 days) Persistent Symptoms |
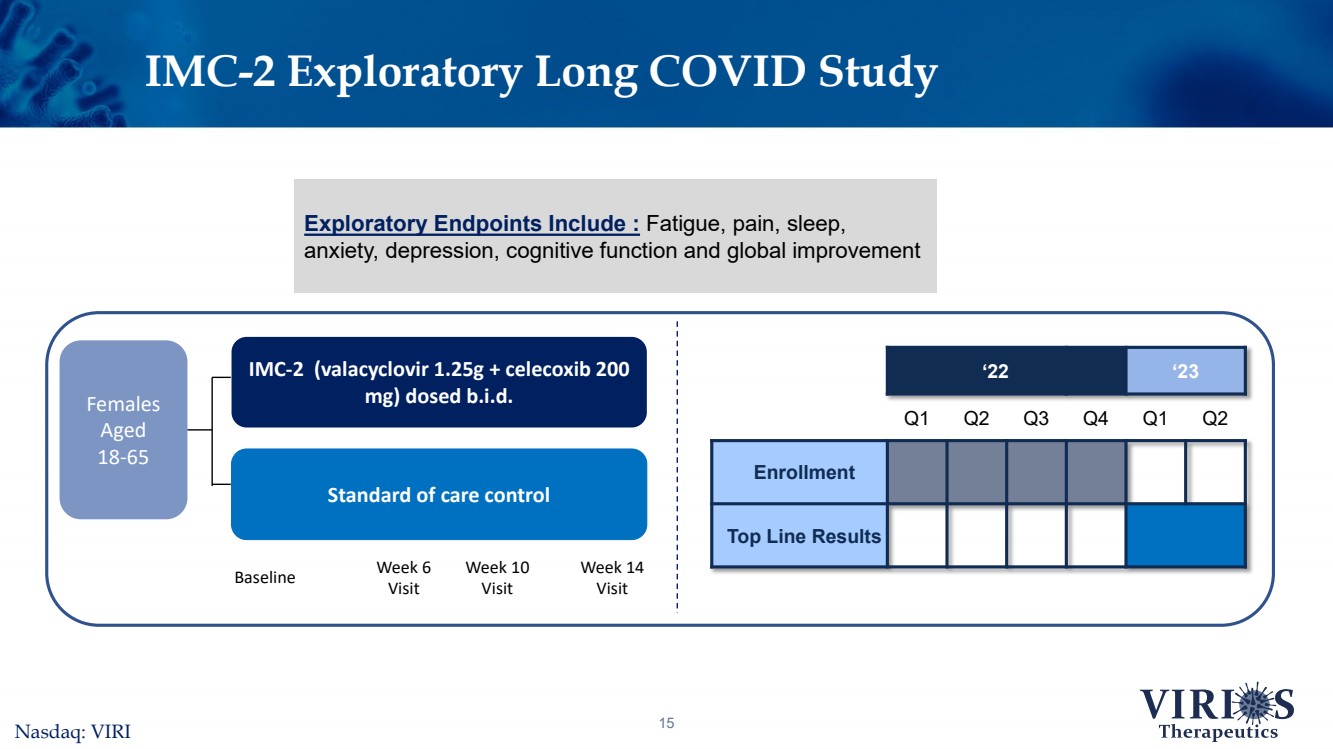
| IMC-2 Exploratory Long COVID Study15Nasdaq: VIRI ‘22‘23Q1Q2Q3Q4Q1Q2EnrollmentTop Line Results Females Aged 18-65 IMC-2 (valacyclovir 1.25g + celecoxib 200 mg) dosed b.i.d. Standard of care control Week 6VisitWeek 10VisitWeek 14Visit Baseline Exploratory Endpoints Include : Fatigue, pain, sleep, anxiety, depression, cognitive function and global improvement |
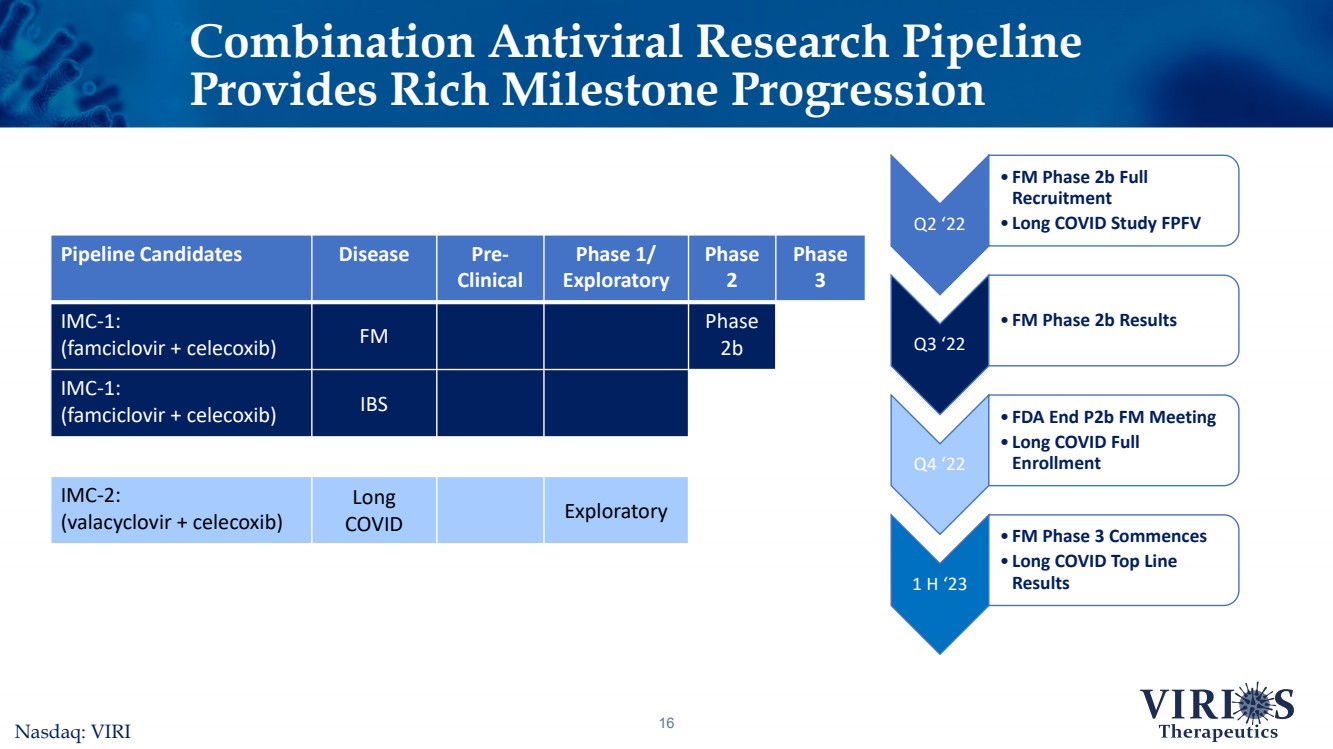
| Combination Antiviral Research Pipeline Provides Rich Milestone Progression16Nasdaq: VIRI Pipeline CandidatesDiseasePre-ClinicalPhase 1/ ExploratoryPhase 2Phase 3IMC-1:(famciclovir + celecoxib)FMPhase 2bIMC-1:(famciclovir + celecoxib)IBSIMC-2: (valacyclovir + celecoxib)Long COVIDExploratory Q2 ‘22 •FM Phase 2b Full Recruitment•Long COVID Study FPFV Q3 ‘22 •FM Phase 2b Results Q4 ‘22 •FDA End P2b FM Meeting•Long COVID Full Enrollment 1 H ‘23 •FM Phase 3 Commences•Long COVID Top Line Results |
| Strong IP Portfolio with 21 Issued Patents17Four IssuedUSIMC-1Patents•Two “CompositionofMatter”Patents•Drug-combinationof famciclovir andcelecoxib•Synergistic combination for total daily dose of famciclovir and celecoxib•Two “Method-of-Use”Patents•Famciclovir+celecoxibforthetreatmentofFM (fibromyalgia), CFSorIBS•Method ofdispensingfamciclovir+celecoxibina regimentotreatFunctionalSomaticSyndromeconditionsSix IssuedForeignIMC-1Patents•EuropeanPatentvalidated in 18 countries•Japan, Australia, China, Koreaand Canada Eight USPatentsCoveringOtherAnti-ViralCombinations•Various combinations of acyclovir, meloxicam, diclofenac, famciclovir, valacyclovir, celecoxibNasdaq: VIRI |
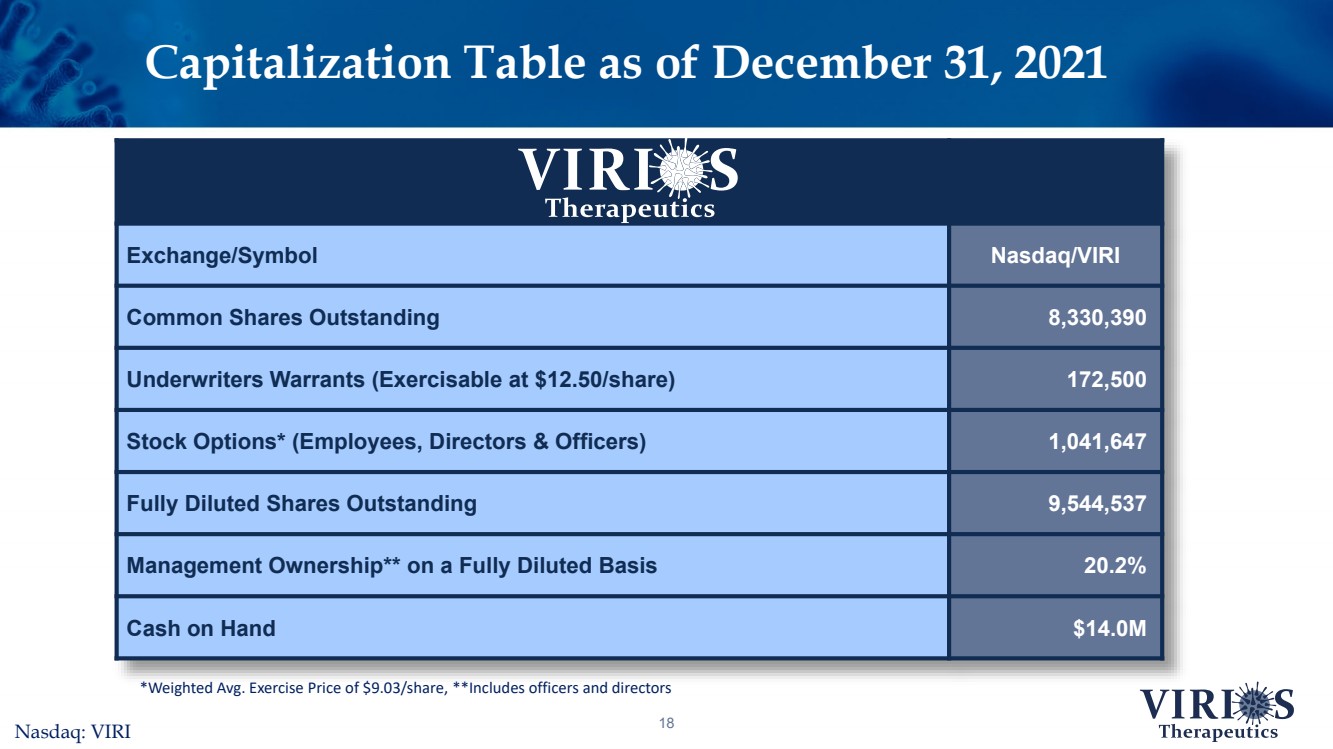
| Capitalization Table as of December 31, 202118 Exchange/SymbolNasdaq/VIRICommon Shares Outstanding8,330,390Underwriters Warrants (Exercisable at $12.50/share) 172,500Stock Options* (Employees, Directors & Officers)1,041,647Fully Diluted Shares Outstanding9,544,537Management Ownership** on a Fully Diluted Basis20.2%Cash on Hand$14.0M *Weighted Avg. Exercise Price of $9.03/share, **Includes officers and directorsNasdaq: VIRI |
| Virios Therapeutics Summary19Nasdaq: VIRIFocused on Two Significant Commercial Opportunities: The Large, Dissatisfied Fibromyalgia(FM) Market: 2%-8% of the PopulationLong COVID, a Rapidly Emerging Unmet Need: Impacts an Estimated 100M Post-COVID Patients WorldwideOral IMC-1 (famciclovir + celecoxib) Demonstrated Significant Pain Reduction Benefits and Tolerability Better Than Placebo in P2a FM Trial:Ongoing Phase 2b FM Trial Results in Q3 2022Composition Of Matter IP to 2033First Ever FDA “Fast Track” Review Designation for FM TreatmentOral IMC-2 (valacyclovir + celecoxib) in Ongoing Phase 2a Long COVID Treatment Trial:COVID-19 acutely depletes our T cells, which may allow for reactivation of Herpes virusesImmune dysregulation may re-activate neurotrophic pathogens such as herpes viruses Virios Team and Board of Directors Have Led Development and Commercialization of Many Category Leading Medicines:Includes Two of Three FDA Approved FM Medicines |
| HSV-1virus Thank You!For Additional Information, Contact:Kirin Smith: ksmith@pcgadvisory.com or ir@virios.com 20 www.virios.com |